PGS Testing – Statistics, Success Rate, Results, and Risks
PGS testing (preimplantation genetic screening) is a type of congenital abnormalities detection in embryos before their transfer to the uterus. It consists of analyzing a set of chromosomes to detect anomalies that cause genetic diseases (Patau’s, Down’s syndrome, etc.)
PGS reduces the likelihood of transferring an embryo that has chromosomal abnormalities into the uterus. It increases the patient’s chances of favorable implantation and overall IVF effectiveness.
Why Parents-to-Be Need to Make Genetic Testing
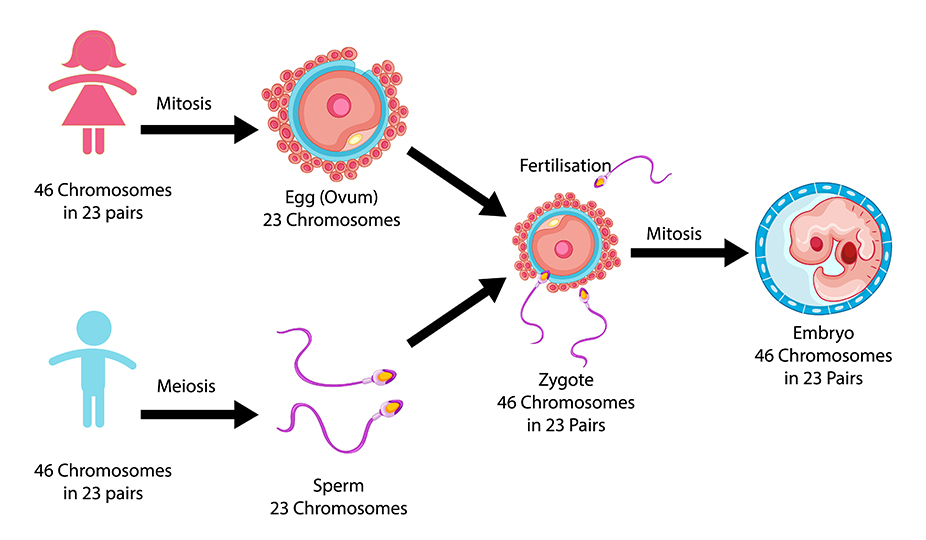
A healthy pregnancy begins with a healthy embryo. Most of the embryos that humans make are abnormal. A normal fetus has 46 chromosomes: 23 from the mother and 23 from the father. If it has more or less than that, it is considered abnormal. It happens in 40-50% of the cases in both older and younger parents-to-be. If abnormal embryos are placed in the uterus they can lead to the following circumstances:
- The embryo fails to implant and does not lead to pregnancy.
- It implants but leads to a later miscarriage. Chromosomal abnormalities are the most common cause of miscarriages.
- It implants and doesn`t miscarry but results in a baby with defects, like Down syndrome.
When Can a Doctor Recommend PGS
Conducting PGS testing is desirable if the patient is in the risk group. This category includes women with recurrent miscarriage, unsuccessful attempts to conceive via ART, patients over 35 years old. Specialists also recommend screening if the couple has a karyotype disorder or a child is born with chromosomal abnormalities.

There is evidence that testing embryos for abnormalities in older women increases the chance for pregnancy, reduces the risk for miscarriage, and reduces the chance for those with congenital disabilities. However, there are different technologies for testing embryos, and not all of them work the same.
How PGS Testing Works in Simple Words
Here is how PGS works. The fertilized egg divides into 2 cells, then 4, 8, and so on. By day 5, there are around 128 distinctive cells. One cluster of cells will become a fetus, another – a placenta.
@sunshine_egg_donation
An embryologist uses a small tool, 1/27 of the diameter of a human hair, to remove a few cells from the cluster that will become a placenta. That sample is overnighted to a lab for testing, while the embryo itself stays safe in the care of a clinic. In the lab, researchers do two things:
- They make millions of copies of the DNA of those cells, so they have plenty to examine.
- Then, they count the chromosomes in each sample removed from each of the developing embryos.
This information is relayed to the clinic, where a fertility doctor can make a weighted and more informed decision about the treatment and transfer a healthy embryo.
That means a dramatically higher chance of starting a long-awaited pregnancy with lower risks of complications. Plus, other healthy embryos can be safely frozen for future use.
PGS Testing Techniques
Doctors make embryo biopsies on the 3rd day (blastomere biopsy) or the 5th day (trophectoderm biopsy) of their development.
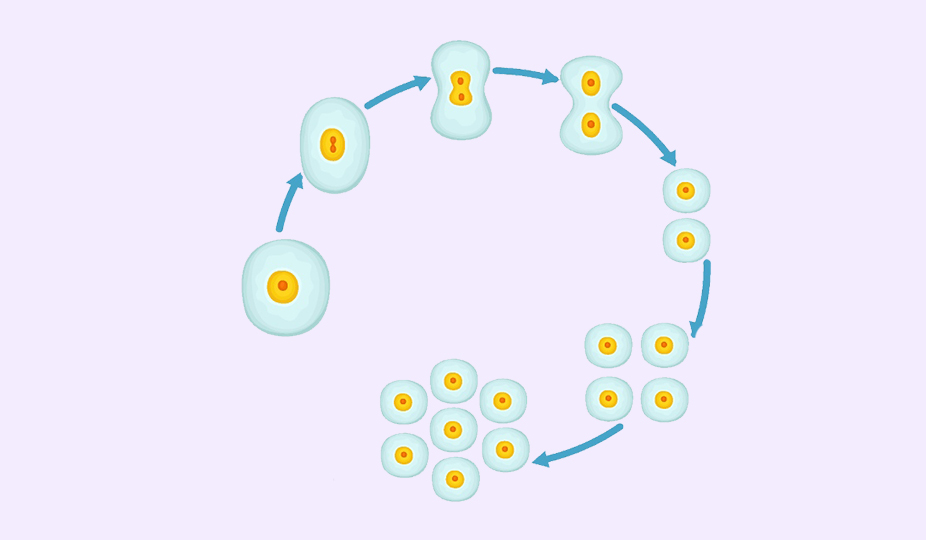
On the third day of development, the embryo usually consists of eight blastomeres (cells). A biopsy at this stage is the separation of one cell. At the same time, there is a risk of damage. Besides, at this stage, embryos have a high level of mosaicism (up to 55%), that is, the presence of at least one cell that differs in chromosome set (genetic composition) from the rest, which causes the risk of false-positive and false-negative diagnostic results.
More common in modern practice is embryo biopsy on the fifth day of development. At this moment, the embryo is already in the blastocyst stage (an early stage of development), which consists of two layers of cells: the intracellular mass and the trophectoderm. Subsequently, the trophectoderm is involved in the formation of the placenta, and the intracellular mass is involved in the formation of the fetus. During the biopsy, the embryologist takes several cells of the trophectoderm, extraembryonic tissue, at once. It significantly reduces the risk of damage, mosaicism, false-positive and false-negative research results. Moreover, it ensures the safety of the procedure and increases diagnostic accuracy.
Known Preimplantation Genetic Testing Technologies
The oldest technology, F.I.S.H., only looks at a few chromosomes. It really has no place for chromosome screening nowadays. Newer technology is an array CGH. It was a great improvement over F.I.S.H. and looked at all 23 pairs of chromosomes. It is still in common use today. The most recent technology is Next Generation Sequencing (NGS). It looks at all chromosomes and has a further ability to identify structural abnormalities and mosaicism. Mosaicism is the case when an embryo has a mixture of different chromosome types. Researchers looked through 1000 cases in which a couple had a single embryo transfer after chromosome testing by either array CGH or NGS. Their findings are really important to us. Then found that am ability of an embryo to implant into the uterus and continue as a normal pregnancy was better with NGS.
At the end of 2017, the world’s most prestigious associations of assisted reproductive medicine agreed on a new glossary (The International Glossary on Infertility and Fertility Care, 2017). The journal Fertility and Sterility highlighted a new term – Preimplantation Genetic Testing or PGT. Scientists added the initials of the type of anomaly that it identifies to the abbreviation to clarify the test’s nature.
At the end of 2017, the world’s most prestigious associations of assisted reproductive medicine agreed on a new glossary (The International Glossary on Infertility and Fertility Care, 2017). The journal Fertility and Sterility highlighted a new term – Preimplantation Genetic Testing or PGT. Scientists added the initials of the type of anomaly that it identifies to the abbreviation to clarify the test’s nature.
PGT-A – Preimplantation Genetic Testing for Aneuploidy
PGT-A corresponds to the old term PGS and serves to detect quantitative abnormalities or aneuploidies. It is ‘preimplantation genetic screening to detect an increase or decrease in the number of chromosomes in embryos.’ If a person has 23 pairs of chromosomes (22 pairs plus a sex pair XX or XY), then in these diseases, the number of chromosomes is disturbed. For example, Down syndrome, when chromosomes of the 21st pair, instead of the normal two, are represented by three copies (trisomy). Besides trisomy 21, the most common aneuploidy in newborns is trisomy 18, trisomy 13, 45, X (Turner syndrome), 47, XXY (Klinefelter syndrome), 47, XYY and 47, XXX.
PGT-SR – Preimplantation Genetic Testing for Structural Diseases
In addition to quantitative abnormalities, there are also structural abnormalities when the structure of one or more chromosomes is abnormal. Those are abnormalities caused by a rupture or incorrect joining of chromosomal segments. Many of the structural abnormalities lead to disease. There are many structural changes: translocations, deletions, duplications, insertions, circular chromosomes, or inversions.
PGT-M – Preimplantation Genetic Testing for Monogenic Diseases
PGT-M detects monosomies, which are inherited diseases caused by a mutation or disruption in the DNA sequence of a single gene. Doctors also call them Mendelian hereditary diseases, as the offspring inherit them following Mendel’s laws. Scientists divide these diseases into three types.
1. Autosomal recessive disorder
To develop the disease requires two copies of a mutated gene in the genome of a child of healthy parents, each of whom carries one copy of the mutated gene. Non-sex chromosomes transmit the disease. The probability of having a child with an autosomal recessive disorder in parents who have one copy of the mutated gene (who do not show any abnormalities) is 25%. An example is a cystic fibrosis or sickle cell anemia.
2. Autosomal dominant disorder
Only one mutated copy of the gene is required for the disease to manifest. Usually, one of the parents of a sick child suffers from this disease, and there is a 50% chance of passing the mutated gene to the offspring. An example is Huntington’s disease or Marfan’s disease.
3. X-related disorder
The X chromosome carries the mutated gene. An example is hemophilia A or fragile X syndrome.
PGT-SR and PGT-M together are equivalent to PGD.
Chelsea Hansen about PGS Testing
PGS Testing FAQ
In 90% of cases, procedures for patients in IVI-assisted reproduction clinics result in pregnancy.
A biopsy of a quality embryo 3-5 days of development does not affect embryo development. At this stage of development, the cells are pluripotent, i.e., interchangeable at the functional level. Thus, the collection of several cells does not cause any harm to the embryo. In contrast, a transfer of a healthy embryo significantly increases the chances of pregnancy after IVF.
First of all, the risk of such an analysis is embryo damage. But it is not very significant. Unfortunately, the study of PGS in IVF has an error rate, and it varies in the range from 8 to 10%. Therefore, there is a possibility of replanting the PGS embryo with an erroneous diagnosis of its quality. When, in fact, it has some pathology.
Many patients fear the cancellation of the transfer within the framework of the IVF protocol after PGS testing. According to the biopsy results, in about 20% of cases, embryologists transfer or completely stop the procedure if the embryos are of bad quality. Ultimately, however, the test guarantees the birth of a healthy child.
The doctor selects several cells from the embryos intended for research (by biopsy). At this stage of development, the selection of cells does not harm their further development. After that, doctors transfer the material to the genetic laboratory. The processing time depends on the type of test, which is recommended and takes more than one day. If the study takes a long time, specialists freeze embryos for further storage. And the transfer is carried out in the next cycle.
Summing It Up
Our bottom line is – until a better technology comes along, next-generation sequencing is the best method for embryo testing and results in a nearly 18% increase in the chance for pregnancy.